The Food and Drug Administration on Friday approved two gene therapies to treat sickle cell disease, one of the which is the first CRISPR/Cas9-based treatment to win regulatory approval in the US.
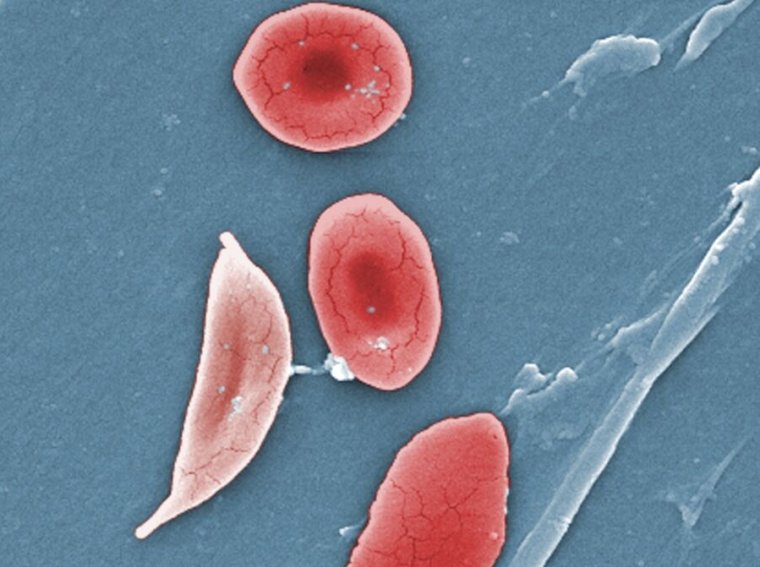
The announcement is a landmark in the treatment of sickle cell disease, a devastating condition in which red blood cells deform into a sickle shape and clog up blood vessels. Sickle cell disease affects around 100,000 people in the US, most commonly African Americans. It leads to anemia, vaso-occlusive events and crises (painful episodes in which small blockages starve tissue of oxygen), strokes, progressive and irreversible organ damage, decreased quality of life, and early death.
Until today, treatments have been limited. A bone marrow transplant from a genetically matched sibling can cure the condition more than 90 percent of the time, but only around 20 percent of people with the disease have such a genetically matched sibling donor. There are also multiple drugs available and supportive care, but these mainly reduce the severity of the disease. The new gene therapy treatments, on the other hand, have shown to be highly effective at preventing vaso-occlusive events and crises.
“Sickle cell disease is a rare, debilitating and life-threatening blood disorder with significant unmet need, and we are excited to advance the field, especially for individuals whose lives have been severely disrupted by the disease, by approving two cell-based gene therapies today,” said Nicole Verdun, director of the Office of Therapeutic Products within the FDA’s Center for Biologics Evaluation and Research, said in the FDA’s announcement.
To grasp how the gene therapies work, it’s useful to grasp what causes sickle cell disease. The central problem is with adult hemoglobin, the iron-containing protein in red blood cells that transports oxygen from the lungs to the rest of the body. In patients with sickle cell disease, there’s a single, small mutation in the gene that encodes hemoglobin. The mutation is a switch of a single nucleotide, or base, (often represented by letters A, C, T, and G). The switch of an A to a T in the genetic code for hemoglobin results in a hemoglobin protein with a valine instead of a glutamic acid at the sixth amino acid position. This transforms normal adult hemoglobin (HbA) to sickle hemoglobin (HbS). In red blood cells, when HbS loses the oxygen it was carrying, it polymerizes with itself, forming strand-admire structures that deform the cell.
Effective edits
The CRISPR/Cas9 therapy approved today, called Casgevy, prevents this deformation by essentially turning on the production of another type of hemoglobin encoded in our genetic blueprints—fetal hemoglobin (HbF). HbF is optimized for pregnancy, transferring oxygen from maternal blood to fetal tissue, and the gene that encodes it is shut off shortly after birth as the body transitions to HbA. About six months after birth, HbF usually makes up just 1 percent to 2 percent of hemoglobin in the body.
But, HbF can effectively treat sickle cell disease—the hemoglobin transports oxygen just fine in adults, and it doesn’t polymerize. Moreover, when it’s mixed with HbS, it gets in the way of the mutated protein polymerizing with itself, preventing it from forming structures that deform red blood cells.
Casgevy turns on HbF with the CRISPR/Cas9 system, a gene-editing system initially swiped from bacteria that snips DNA using an enzyme (a nuclease) called Cas9. Cas9 can be targeted to specific stretches of DNA using a short RNA guide sequence. In Casgevy, the CRISPR/Cas9 system is targeted to snip a gene encoding a protein called BCL11A, which controls other genes, aka a transcription factor. The BCL11A transcription factor is the protein responsible for shutting off the gene for HbF shortly after birth as the body transitions to the adult version. With the CRISPR/Cas9 snip, BCL11A is shut off, and HbF production can resume.
For patients being treated, this process involves first harvesting their bone marrow stem cells, which then get CRISPR-ed in a specialized lab. Meanwhile, the patients acquire chemotherapy to conclude bone marrow cells to make way for the gene-edited cells that are then put back in. Of 31 patients treated with Cagevy and followed for at least 24 months, 29 (93.5 percent) went at least 12 consecutive months without a vaso-occlusive crisis.
The other gene therapy approved by the FDA today is Lyfgenia, which used a Lentiviral vector to insert genes into the human genome. In this case, the system delivers the genetic code for a modified type of hemoglobin that is designed to be anti-sickling, called HbAT87Q. Among 32 patients treated with Lyfgenia, 28 (88 percent) were free of vaso-occlusive events for between six to 18 months after treatment.
Both gene therapies are approved for patients ages 12 years and up.

