Andy/iStock via Getty Images
In my third edition of my CRISPR series (see article 1 (an overview of CRISPR-Cas9) and article 2 (a comparison of CRSP and NTLA)), I want to focus on Beam Therapeutics (NASDAQ:BEAM), which is a favorite amongst investors because it uses what many believe to be a better version of gene editing, namely base editing.
Base editing is a novel approach to gene editing that allows for the direct, irreversible conversion of one DNA base pair into another, at a specific genomic location, without creating a double-stranded break in the DNA. This technology offers several advantages:
-
Precision: Base editing enables precise changes at the DNA level, allowing for the correction of point mutations that are the root provoke of many genetic diseases.
-
Reduced Off-target Effects: Since base editors do not rely on creating double-strand breaks in the DNA (unlike traditional CRISPR-Cas9 methods), they potentially reduce the risk of unintended genetic changes or off-target effects. The key word here is potentially because this method is not yet tested in the clinic.
-
Versatility: This technology can be used to correct a wide range of point mutations, making it applicable to many genetic disorders. This solves the limitations of being able to only edit one mutation with legacy CRISPR-Cas9.
-
Targeting Capability: Base editors are designed to target specific DNA sequences, offering a high degree of specificity in gene editing.
Beam Therapeutics’ focus on base editing technology represents a significant advancement in the field of gene therapy, allowing for more precise and potentially safer genetic modifications compared to earlier gene editing methods. This technology is particularly promising for treating genetic diseases caused by specific point mutations.
Before we dive into BEAM’s pipeline, I thought it would be helpful to go into more depth on exactly how base editing works.
How Exactly Does Base Editing Work?
Base editing works by directly converting one DNA base into another without making double-stranded breaks in the DNA.
As a refresher, a DNA base is a fundamental component of the DNA molecule, which carries genetic information in living organisms. DNA consists of two long strands that form a double helix, and these strands are made up of smaller units called nucleotides.
Each nucleotide comprises a sugar molecule (deoxyribose), a phosphate group, and a nitrogenous base. It’s the sequence of these bases along the DNA strand that encodes genetic information.
Here is an illustration for those of us that appreciate visuals:
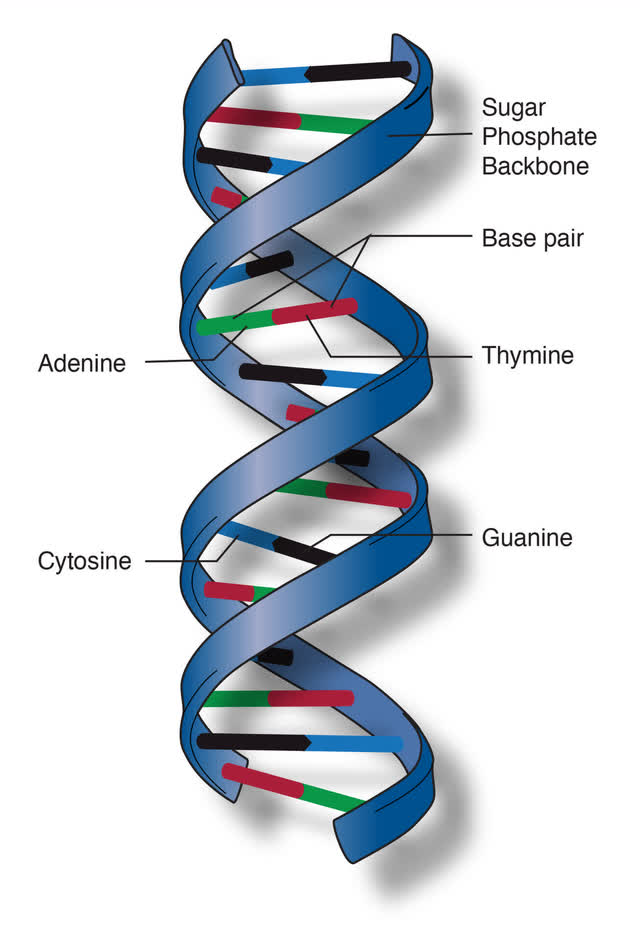
DNA Base – Story MD (DNA Base)
As you can see in the illustration, there are 4 types of bases:
- Adenine (A): A purine base that pairs with Thymine (T) in DNA through two hydrogen bonds.
- Thymine (T): A pyrimidine base that pairs with Adenine (A).
- Cytosine (C): Another pyrimidine base, which pairs with Guanine (G) through three hydrogen bonds.
- Guanine (G): A purine base that pairs with Cytosine (C).
The base pairing (A with T, C with G) is specific and is known as complementary base pairing. This pairing is crucial for the replication of DNA, as each strand serves as a template for creating a new complementary strand.
The order, or sequence, of these bases determines the information available for building and maintaining an organism, similar to the way letters of the alphabet appear in a certain order to form words and sentences.
Understanding DNA bases is essential for grasping how genetic information is stored and transmitted in living organisms, and it helps instruct researchers on what mutations to edit in gene editing.
Components of Base Editing
Base editing uses a modified form of the CRISPR-Cas system, where the Cas enzyme (appreciate Cas9) is engineered to lose its ability to create double-stranded breaks in DNA.
Think of legacy CRISPR-Cas9 as scissors cutting an entire piece of paper in half. Base editing doesn’t make the cut across the entire piece of paper.
In base editing, the Cas enzyme binds to a specific target site in the DNA. The enzyme is fused to the modified Cas protein.
In the context of base editing, the “modified Cas protein” refers to an engineered version of the Cas (CRISPR-associated) protein, which is a key component of the CRISPR-Cas system used for gene editing.
The modification typically involves altering the Cas protein so that it no longer cuts both strands of the DNA helix, which is its natural function in the CRISPR-Cas9 system. Instead, the modified Cas protein is designed to only bind to the DNA at the target site without causing double-strand breaks.
Similar to standard CRISPR-Cas9, base editing also utilizes a guide RNA to direct the Cas-deaminase complex to the correct DNA sequence.
In base editing, the modified Cas protein is fused to a base deaminase enzyme. The deaminase enzyme directly alters the nitrogenous bases of the DNA (e.g., converting cytosine to uracil).
The modified Cas protein’s role is to guide the deaminase to the correct part of the genome, as determined by the guide RNA, and to hold the DNA in the correct conformation to allow the deaminase to perform its function.
This modification is crucial for base editing as it allows for precise, targeted changes to the DNA bases without the risks associated with creating double-strand breaks, such as unintended insertions, deletions, or rearrangements in the genome.
This approach in theory represents a significant advancement in the precision and safety of genome editing technologies.
This is a good drawing I came across that shows all of the editing types including gene therapy in the eye. It might be helpful for you to visualize the differences.
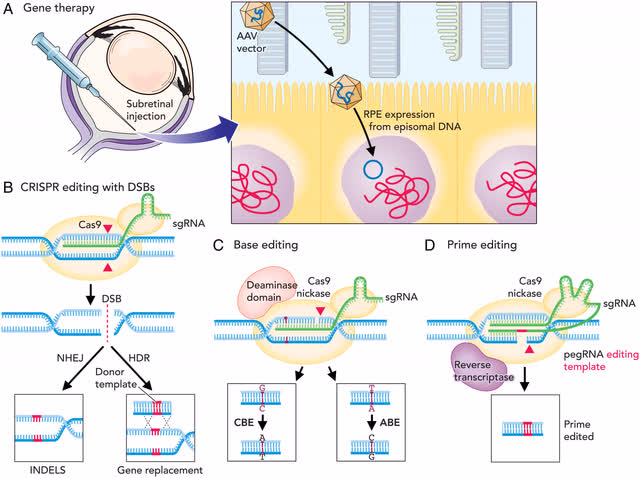
DNA editing in the eye (DNA editing in the eye (PNAS))
RECAP – The Process, Applications, and Limitations
- Targeting the DNA: The gRNA guides the Cas-deaminase complex to a specific DNA sequence.
- Base Conversion: Once bound, the deaminase enzyme chemically converts one base to another. For instance, a Cytosine (C) base can be converted to Uracil (U), which functions appreciate Thymine (T) during DNA replication. Thus, a C•G base pair can be effectively changed to a T•A base pair.
- DNA Replication: When the cell replicates its DNA, the DNA polymerase reads the Uracil as Thymine, cementing the change from a C•G to a T•A base pair.
Applications and Limitations
- Applications: Base editing is useful for correcting point mutations that provoke genetic diseases.
- Limitations: While more precise than traditional CRISPR-Cas9, base editing has its own limitations. It can only make certain types of base pair changes, and there’s still a risk of off-target effects, though generally lower than CRISPR-Cas9.
Beam Therapeutics Pipeline
With a general understanding of the technology underpinning BEAM, let’s proceed on to their pipeline, financials, and my opinion on them.
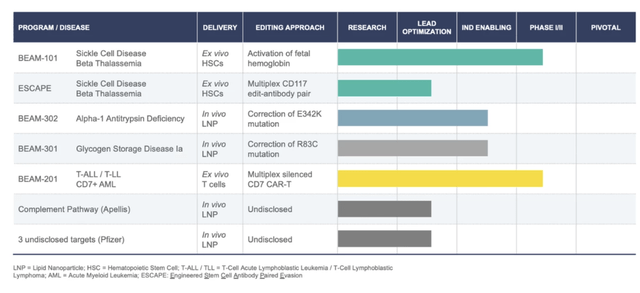
Beam Therapeutics Pipeline (Beam Therapeutics Pipeline)
BEAM-101
BEAM-101 is an investigational therapy that produces base edits designed to potentially alleviate the effects of sickle cell disease by mimicking genetic variants seen in individuals who have hereditary persistence of fetal hemoglobin.
It is delivered via electroporation and it is an ex-vivo cell therapy in which cells are collected from a patient, edited, and then infused back into the patient.
Given that CRISPR Therapeutics (CRSP) direct drug is in the same space and is about to be approved, I think it’s a tough space for BEAM to contend in given how far behind they are. It will likely be another 2-3 years until this is up for approval, if not longer.
BEAM-201
BEAM-201 is a multiplex base edited anti-CD7 CAR-T cell investigational therapy for relapsed and refractory T-cell acute lymphoblastic leukemia and T-cell lymphoblastic lymphoma, a severe disease affecting children and adults.
It is delivered via electroporation and BEAM is using multiplex base editing to create donor-derived CAR-T cells by simultaneously silencing multiple target genes.
BEAM dosed their first patient in September 2023 for this therapy and it is in phase 1 with the FDA so it is still a long ways off from being approved.
BEAM-301
The company initiated IND-enabling studies for BEAM-301, a treatment targeting glycogen storage disease 1a (GSDIa). This liver-targeting treatment uses lipid nanoparticle base-editing reagent formulations and is notable for its potential as the first gene-editing product to directly correct a genetic mutation in vivo. This is not in clinical trials yet.
BEAM-302
This treatment is developed for Alpha-1 antitrypsin deficiency and involves a base editing approach for correcting the E342K mutation. BEAM-302 demonstrates Beam’s potential in addressing challenging target sites through base editing. This is not in clinical trials yet.
Balance Sheet
BEAM cash burn: ~$400M per year
BEAM cash: $1.0 Billion
BEAM cash runway: 2-3 years
Cash doesn’t seem to be a near term concern for BEAM; however, they’re more than 2-3 years away from commercial approval of any of their therapies so they will likely need to raise money at some point.
Conclusion
Base editing seems very promising. One of the concerns as I mentioned in my other articles about CRISPR is off target edits (see below) and it seems appreciate base editing may minimize these issues (tbd though because clinical trials are just underway).

Off target edits (Off target edits)
As I mentioned before, it’s likely that gene editing doesn’t really kick into gear until the 2030s.. That doesn’t mean that these will be bad investments but it does mean you will have to sit through at least 2 more dilutions along the way.
Considering that these therapies could be truly life altering, the potential is for these companies to become giants (is $500B market cap potential possible?) and even if it takes 10 years with a couple of rounds of dilutive capital raising along the way, you’re talking about the potential for 30-100X returns (key word is potential as it is far from guaranteed). The CAGR of 30X over 10 years is >40%.
BEAM is battling a bit of an uphill battle, though, because their indications are competing with the first wave of CRISPR therapies. So they would need to be meaningfully better and lower risk to convince regulators and patients to use it.
For these reasons, I haven’t invested in BEAM stock, but I will be watching it and keeping an eye on their clinical trial readouts.



