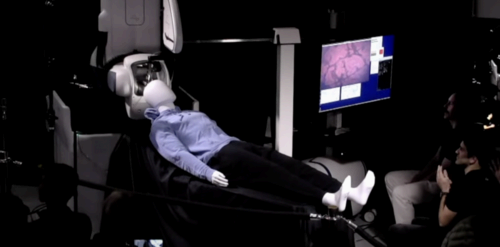
Billionaire Elon Musk posted on social media late Monday that his brain-computer interface company, Neuralink, implanted an experimental device into the brain of a human for the first time Sunday.
According to Musk’s posts, which appeared on the platform formerly known as Twitter, the recipient is “recovering well,” and the results in the first 24 hours show “promising neuron spike detection.” Neuralink implanted the person with a device called Telepathy, which is intended to allow users to control devices, such as phones and computers, only by thinking.
The implantation comes around eight months after the company announced that the Food and Drug Administration had finally granted a long-sought approval to begin its first human trial. Recruitment for the trial began in September. Musk had claimed to be nearing trials starting as early as 2020, but the FDA reportedly denied approval in 2022, citing a list of dozens of “deficiencies” and safety concerns that Neuralink had yet to address.
According to a brochure on its website, Neuralink’s trial, called the PRIME (Precise Robotically Implanted Brain-Computer Interface) Study, is designed to test both the brain-computer interface implant and the surgical robot the company uses to delicately implant the device directly into brain tissue involved in movement intention. People eligible to volunteer included people who had lost the use of all four limbs due to a spinal cord injury or amyotrophic lateral sclerosis (ALS).
Neuralink did not immediately respond to Ars’ request for comment. As of the time of publication, the company had not released any news or information about the trial’s progress or the first human recipient. It also appears that the company has still not registered its clinical trial on the federal registry for clinical trials (clinicaltrials.gov). However, it did repost Musk’s postings from Monday on its own social media account.
Neuralink’s first human implantation comes after a string of problems and federal scrutiny, in addition to the delayed start of its clinical trial. The company is reportedly under federal investigation for alleged animal abuse, including accusations from current and former Neuralink employees of “hack job” surgeries and needless suffering and deaths of pigs and monkeys. Last week, Reuters reported that the company was fined for violating US Department of Transportation (DoT) rules regarding the movement of hazardous materials, including xylene, a toxic and flammable solvent sometimes used in tissue processing. Even with the progress on its clinical trial, Neuralink is still considered far behind competitors, such as Blackrock Neurotech, which has years of clinical work under its belt.

